Abstract
Background: Advances in the treatment of multiple myeloma (MM) have markedly improved its prognosis. Novel agents such as bortezomib (BTZ) and lenalidomide (LEN) have largely replaced traditional chemotherapy due to their greater efficacy and better tolerability. However, the optimal sequencing of these treatments is still unclear as head-to-head trials are lacking. In SWOG S0777, combination BTZ, LEN, and dexamethasone (DEX) for first-line therapy induced higher remission rates and improved overall survival (OS) by 11 months (75 months compared to 64 months, p = 0.025) over LEN and DEX alone (Durie, et al, Lancet, 2017). However, combination treatment was more toxic with over twice as many patients discontinuing treatment (23% compared to 10%). The study did not analyze the cost implications of combination therapy, which is likely substantial as the cost of either BTZ or LEN alone is. The regimen's impact on healthcare costs and quality-of-life (QOL) should be considered when evaluating its utility.
Objective: In this study, we aim to compare the costs and benefits of BTZ, LEN, and BTZ/LEN combination therapy for first-line MM treatment using real-world cost data from the SEER-Medicare database.
Methods: All patients diagnosed with MM from 2007-2013 were identified in the SEER-Medicare database. Patients were considered eligible for the analysis if they were over 65 years of age at diagnosis, had Medicare Part A and B for 12 months prior to and Part D at the time of diagnosis, had an overall survival of at least 12 months, and received induction therapy with BTZ, LEN, or BTZ/LEN combination therapy. Patients who received any additional anti-MM therapy or underwent stem cell collection or transplantation within 6 months of MM diagnosis were excluded.
All direct medical costs, including inpatient, outpatient, and prescriptions, were captured for the first 84 days of treatment, which corresponds to 3 4-week cycles of LEN or 4 3-week cycles of BTZ or BTZ/LEN. Costs included both those paid by Medicare and patient copays, coinsurance, and deductibles and were adjusted for inflation to 2014 dollars using the medical services section of the Consumer Price Index. Number of visits and length of inpatient hospital stays were also compared. Bivariate analysis was performed using Chi-square or ANOVA; multivariate analysis using linear regression.
Results: 1162 patients were included in the final analysis, 542 in the BTZ cohort, 446 in the LEN cohort, and 174 in the BTZ/LEN cohort. The cohorts were similar in gender and racial composition; however, the BTZ/LEN cohort was younger with a median age of 72 years compared to 76 years for BTZ and LEN (p < 0.0001) and was less likely to have indicators of poor performance status (10% compared to 19% and 18%, respectively, p = 0.0348). The mean comorbidity index of the BTZ cohort was higher than the LEN and BTZ/LEN cohorts (1.9 compared to 1.3, p < 0.0001).
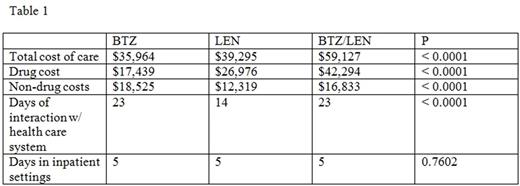
The average drug cost for BTZ, LEN, and BTZ/LEN therapy was $17,439, $26,976, and $42,294 (p < 0.0001), respectively, and the total cost of care was $35,964, $39,295, and $59,127 (p < 0.0001). Controlling for variables such as age, comorbidities, and performance status, LEN was associated with $4,051 greater total cost of care (p = 0.0003) and BTZ/LEN with $23,213 greater total cost of care (p < 0.0001) compared to BTZ. The LEN cohort had an average of 14 days of interaction with the medical system compared to 23 days for each of the BTZ containing cohorts (p < 0.0001); the average number of days spent in inpatient settings was 5 in each cohort (p = 0.7602). OS in the BTZ and LEN cohorts was similar but BTZ/LEN was associated with a 36% decrease in hazard ratio (aHR 0.64, 95% CI 0.44-0.94) for mortality on multivariate analysis.
Conclusions: First-line BTZ/LEN combination therapy is associated with superior OS but with increased drug costs. LEN and BTZ as single-agents had similar survival outcomes, and while LEN had higher drug costs, it is an oral formulation and had lower non-drug costs and reduced frequency of visits which improve its cost-utility. Newer agents such as carfilzomib, ixazomib, and daratumumab are increasingly being used for first-line therapy. We were unable to estimate the costs of such therapies because data is currently lacking. However, as drug costs are the largest driver of total cost of care, these newer agents will likely further increase the economic burden of MM treatment.
Wildes: Carevive Systems Inc: Consultancy; Janssen: Consultancy. Vij: Celgene, Onyx, Takeda, Novartis, BMS, Sanofi, Janssen, Merck: Consultancy; Takeda, Onyx: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal